Kevzara
What is Kevzara (Sarilumab)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The main purpose of this study is to: * Learn about the safety of ubamatamab and to find out what dose of ubamatamab can be given alone or with cemiplimab to patients with ovarian cancer or cancer of the uterus * The study will also look at the levels of ubamatamab and/or cemiplimab in the body and measure how well the body can remove the study drug(s). This is called pharmacokinetics * The study ...
Summary: This study is researching an experimental drug called ubamatamab, also referred to as study drug. The study is focused on patients who have advanced ovarian cancer. The aim of the study is to see how safe, tolerable, and effective the study drug is on its own and in combination with other anti-cancer drugs (bevacizumab, cemiplimab, fianlimab and a standard chemotherapy drug, pegylated liposomal do...
Objective: To describe the pharmacokinetic (PK) profile of sarilumab in patients aged 1-17 years with Systemic Juvenile Idiopathic Arthritis (sJIA) in order to identify the dose and regimen for adequate treatment of this population. Secondary Objective: To describe the pharmacodynamics (PD) profile, the efficacy, and the long term safety of sarilumab in patients with sJIA.
Related Latest Advances
Brand Information
- Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Patients should be tested for latent tuberculosis before KEVZARA use and during therapy. Treatment for latent infection should be initiated prior to KEVZARA use.
- Invasive fungal infections, such as candidiasis, and pneumocystis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
- Bacterial, viral and other infections due to opportunistic pathogens.
- Serious infections
- Neutropenia, thrombocytopenia, elevated liver enzymes, lipid abnormalities
- Gastrointestinal perforation
- Immunosuppression
- Hypersensitivity reactions

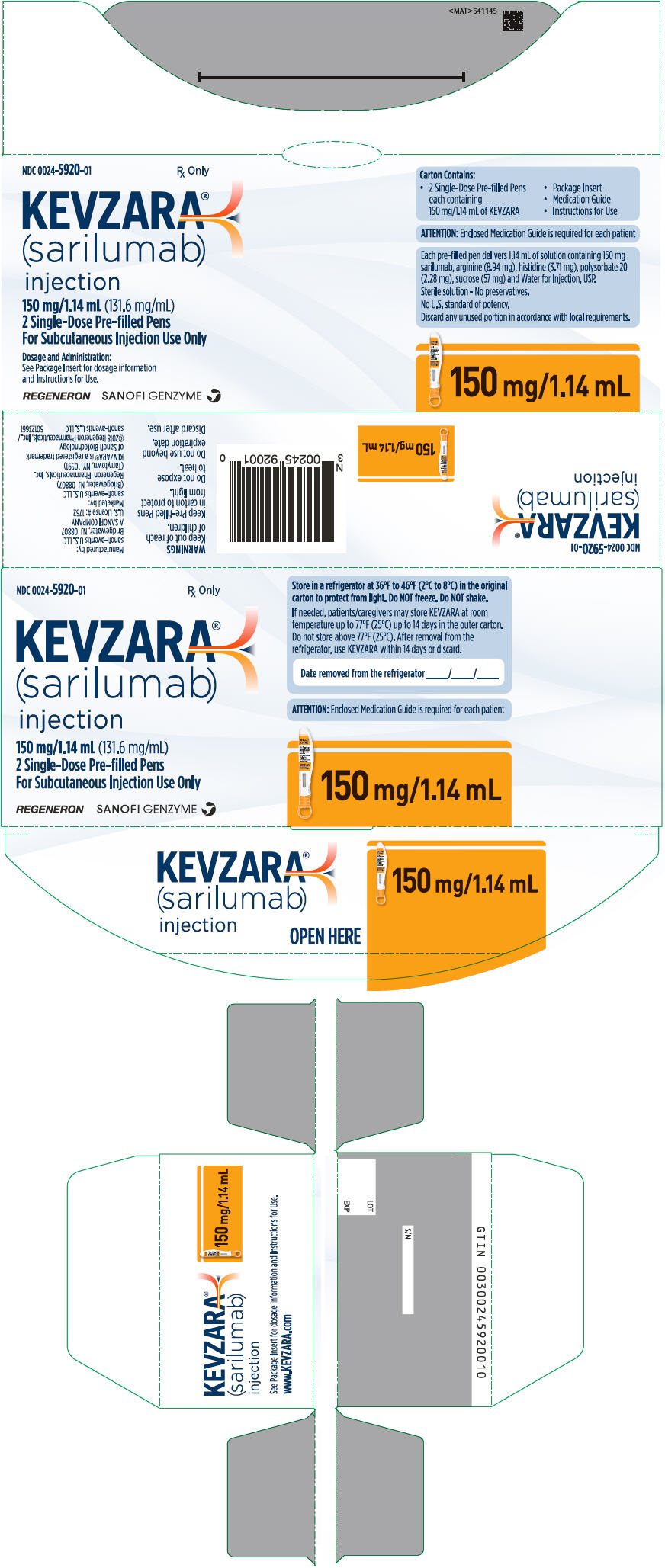
- A new KEVZARA 150 mg/1.14 mL pre-filled syringe
- Alcohol wipe
- Cotton ball or gauze
- Sharps disposal container. See "
- Put the used syringe in an FDA-cleared sharps disposal container right away after use.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not reuse the syringe.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
sanofi

- A new KEVZARA 200 mg/1.14 mL pre-filled syringe
- Alcohol wipe
- Cotton ball or gauze
- Sharps disposal container. See "
- Put the used syringe in an FDA-cleared sharps disposal container right away after use.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not reuse the syringe.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
sanofi
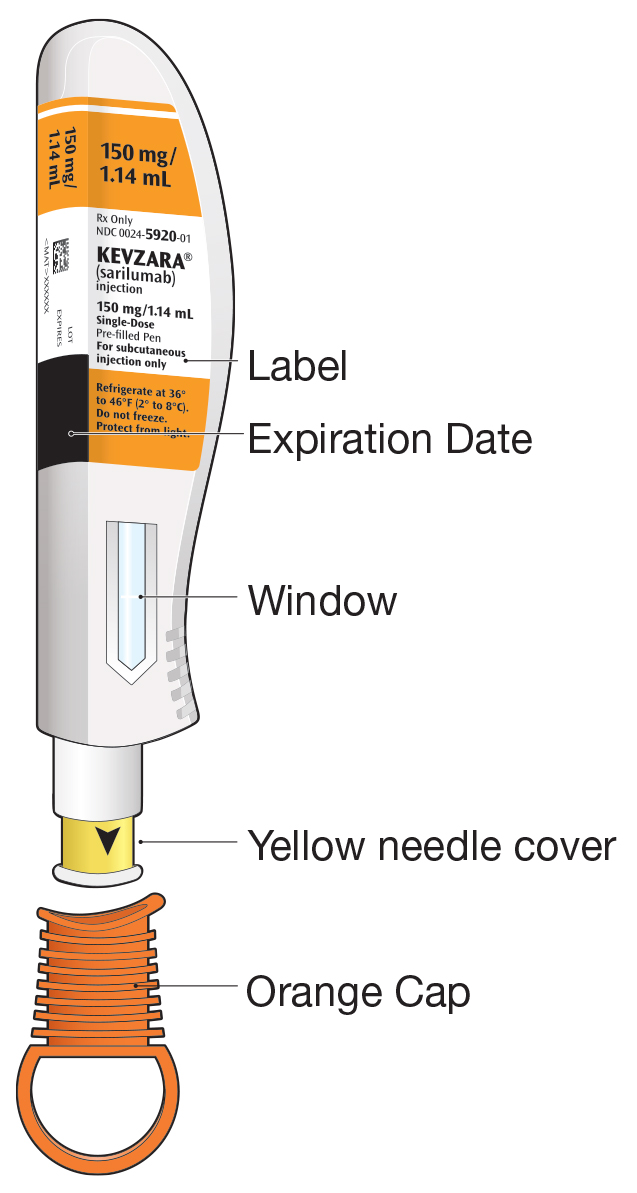
- An unused KEVZARA 150 mg/1.14 mL pre-filled pen
- Alcohol wipe
- Cotton ball or gauze
- Sharps disposal container. See "
- Put the used pen in an FDA-cleared sharps disposal container right away after use.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not reuse the pen.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
sanofi

- An unused KEVZARA 200 mg/1.14 mL pre-filled pen
- Alcohol wipe
- Cotton ball or gauze
- Sharps disposal container. See "
- Put the used pen in an FDA-cleared sharps disposal container right away after use.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not reuse the pen.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
sanofi