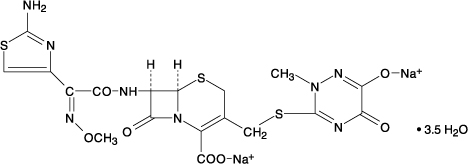
CefTRIAXone
View Brand InformationWhat is CefTRIAXone?
Top Local Experts
There are no experts for this drug
Related Clinical Trials
Summary: This study will determine whether patients who have been infected with the Lyme bacteria, Borrelia burgdorferi, and treated with antibiotics still have the bacteria alive inside them and whether it is causing their symptoms. The information from this study may serve as a basis for developing stringent diagnostic criteria for Lyme disease and the establishment of future treatment trials. Individual...
Summary: The goal of this clinical trial is to learn if an FDA approved drug, Ceftriaxone, given intermittently, can treat people between 18 and 75 years old with a history of Lyme disease, who are still experiencing persistent or returning symptoms after they have completed treatment. The main questions it aims to answer are: * Will giving Ceftriaxone approximately every 5 days for 6 weeks be safe and wel...
Summary: An anterior cruciate ligament (ACL) tear is one of the knee joint's most common soft tissue injuries \[1\]. It is frequently injured in non-contact and some contact competition sports and even during ordinary life activities. With an annual incidence of 68.6 per 100,000 person-years, ACL tears remain a common orthopedic injury \[2\]. Females are two to eight times more likely to develop ACL tears ...
Related Latest Advances
Brand Information
- Gram-negative Bacteria
Acinetobacter calcoaceticus
Enterobacter aerogenes
Enterobacter cloacae
Escherichia coli
Haemophilus influenzae
Haemophilus parainfluenzae
Klebsiella oxytoca
Klebsiella pneumoniae
Moraxella catarrhalis
Morganella morganii
Neisseria gonorrhoeae
Neisseria meningitidis
Proteus mirabilis
Proteus vulgaris
Pseudomonas aeruginosa
Serratia marcescens - Gram-positive Bacteria
Staphylococcus aureus
Staphylococcus epidermidis
Streptococcus pneumoniae
Streptococcus pyogenes
Viridans group streptococci - Anaerobic Bacteria
Bacteroides fragilis
Clostridium species
Peptostreptococcus species
- Gram-negative Bacteria
Citrobacter diversus
Citrobacter freundii
Providencia species (including Providencia rettgeri)
Salmonella species (including Salmonella typhi)
Shigella species - Gram-positive Bacteria
Streptococcus agalactiae - Anaerobic Bacteria
Porphyromonas (Bacteroides) melaninogenicus
Prevotella (Bacteroides) bivius
- Advise patients that neurological adverse reactions could occur with ceftriaxone for injection use. Instruct patients or their caregivers to inform their healthcare provider at once of any neurological signs and symptoms, including encephalopathy (disturbance of consciousness including somnolence, lethargy, and confusion), seizures, myoclonus, and nonconvulsive status epilepticus, for immediate treatment, or discontinuation of ceftriaxone for injection (see WARNINGS and PRECAUTIONS).
- Patients should be counseled that antibacterial drugs including ceftriaxone for injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., common cold).
- When ceftriaxone for injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ceftriaxone for injection or other antibacterial drugs in the future.
- Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
