Brand Name
Vivjoa
Generic Name
Oteseconazole
View Brand Information FDA approval date: July 11, 2022
Classification: Azole Antifungal
Form: Capsule
What is Vivjoa (Oteseconazole)?
VIVJOA™ is an azole antifungal indicated to reduce the incidence of recurrent vulvovaginal candidiasis in females with a history of RVVC who are NOT of reproductive potential.
Approved To Treat
Top Local Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
VIVJOA (oteseconazole)
1INDICATIONS AND USAGE
1.1Vulvovaginal Candidiasis
VIVJOA is indicated to reduce the incidence of recurrent vulvovaginal candidiasis (RVVC) in females with a history of RVVC who are NOT of reproductive potential [see Warnings and Precautions (5.1), Use in Specific Populations (8.3), and Clinical Studies (14)].
1.2Usage
If specimens for fungal culture are obtained prior to therapy, antifungal therapy may be instituted before the results of the cultures are known. However, once these results become available, antifungal therapy should be adjusted accordingly.
2DOSAGE AND ADMINISTRATION
2.1Dosage Overview and Important Administration Instructions
There are two recommended VIVJOA dosage regimens: a VIVJOA-only regimen and a Fluconazole/VIVJOA regimen. Use one of the following two dosage regimens:
- VIVJOA-only dosage regimen [see Dosage and Administration (2.2)]
- Fluconazole/VIVJOA dosage regimen [see Dosage and Administration (2.3)].
Administer VIVJOA orally with food [see Clinical Pharmacology (12.3)]. Swallow the capsules whole. Do not chew, crush, dissolve, or open the capsules.
2.2VIVJOA-only Dosage Regimen
For the VIVJOA-only dosage regimen:
- On Day 1: Administer VIVJOA 600 mg (as a single dose), then
- On Day 2: Administer VIVJOA 450 mg (as a single dose), then
- Beginning on Day 14: Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 2 through 12).
2.3Fluconazole/VIVJOA Dosage Regimen
For the Fluconazole/VIVJOA dosage regimen, prescribe fluconazole and:
- On Day 1, Day 4, and Day 7: Administer fluconazole 150 mg orally, then
- On Days 14 through 20: Administer VIVJOA 150 mg once daily for 7 days, then
- Beginning on Day 28: Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 4 through 14).
3DOSAGE FORMS AND STRENGTHS
VIVJOA Capsules: 150 mg of oteseconazole in lavender hard gelatin capsules imprinted with OTE 150 in black ink.
Fluconazole is not supplied in the carton.
4CONTRAINDICATIONS
VIVJOA is contraindicated in:
- Females of reproductive potential [see Warnings and Precautions (5.1) and Use in Specific Populations (8.3)]
- Pregnant and lactating women [see Warnings and Precautions (5.1), and Use in Specific Populations (8.1, 8.2)]
- Patients with known hypersensitivity to oteseconazole.
5WARNINGS AND PRECAUTIONS
5.1Embryo-Fetal Toxicity
VIVJOA is contraindicated in females of reproductive potential, and in pregnant and lactating women. Based on animal studies, VIVJOA may cause fetal harm. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks. Ocular abnormalities were observed in the offspring of pregnant rats dosed at 7.5-mg/kg/day during organogenesis through lactation in pre and postnatal developmental studies. The observed ocular abnormalities included cataracts, opacities, exophthalmos/buphthalmos, optic nerve/retinal atrophy, lens degeneration and hemorrhage. Ocular abnormalities occurred at doses about 3.5 times the steady state clinical exposure seen with patients being treated for RVVC. Advise patients that VIVJOA is contraindicated in females of reproductive potential, and in pregnant and lactating women because of potential risks to a fetus or breastfed infant [see Use in Specific Populations (8.1, 8.2, 8.3)].
6ADVERSE REACTIONS
6.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of one drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 580 patients were treated with VIVJOA in three clinical trials (Trial 1, Trial 2, and Trial 3) [see Clinical Studies (14)]. Patients in the clinical trials were women with RVVC who received VIVJOA treatment for 12 weeks. The mean age of the patient population was 34 years (range:16-78 years), with 84% of patients aged 18-44 years and 16% of patients aged 45 years and older. Although females of reproductive potential were included in the clinical safety data, VIVJOA is contraindicated in females of reproductive potential due to the risk of embryo-fetal toxicity [see Contraindications (4), Warnings and Precautions (5.1), and Use in Specific Populations (8.1, 8.3, 8.4)].
The clinical trials population was 75% (435/580) White, 17% (96/580) Black or African American, 6% (36/580) Asian, and 2% (13/580) Other women. Fifteen percent (86/580) of all women were Hispanic/Latino. Patients enrolled in the induction and maintenance phases of the clinical trials were treated with different VIVJOA dosage regimens versus comparators [see Clinical Studies (14)].
The adverse reaction that led to discontinuation in 1 of 580 (0.2 %) VIVJOA-treated patients was allergic dermatitis. Overall, similar percentages of serious adverse reactions and adverse reactions leading to drug discontinuation were reported across the VIVJOA and comparator patient dosing groups.
The most frequently reported adverse reactions (incidence >2%) among VIVJOA-treated patients in Trial 1, Trial 2 and Trial 3 were headache (includes headache, migraines, sinus headaches) (7.4%) and nausea (3.6%).
7DRUG INTERACTIONS
7.1Effect of VIVJOA on Other Drugs
8USE IN SPECIFIC POPULATIONS
8.1Pregnancy
8.2Lactation
8.3Females of Reproductive Potential
VIVJOA is contraindicated in females of reproductive potential based on animal findings. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks [see Warnings and Precautions (5.1), Use in Specific Populations (8.1) and Clinical Pharmacology (12.3)].
Females who are NOT of reproductive potential are defined as: persons who are biological females who are postmenopausal or have another reason for permanent infertility (e.g., tubal ligation, hysterectomy, salpingo-oophorectomy).
8.4Pediatric Use
VIVJOA is contraindicated in females of reproductive potential. Based on animal studies, VIVJOA may cause fetal harm when administered to a pregnant woman or potential harm to the breastfed infant. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks associated with VIVJOA use [see Contraindications (4), Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.2, 8.3) and Clinical Pharmacology (12.3)].
The safety and effectiveness of VIVJOA have not been established in pre-menarchal pediatric females.
8.5Geriatric Use
Clinical studies of VIVJOA did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
8.6Renal Impairment
No dosage adjustment of VIVJOA is recommended in patients with mild to severe renal impairment (i.e., estimated glomerular filtration rate (eGFR) by the modification of diet in renal disease (MDRD) equation 15-89 mL/min). The effect of end-stage renal disease (eGFR <15 mL/min) on the pharmacokinetics of oteseconazole is unknown. Dialysis is not expected to alter oteseconazole exposures [see Clinical Pharmacology (12.3)].
8.7Hepatic Impairment
No dosage adjustment of VIVJOA is recommended in patients with mild or moderate hepatic impairment (Child-Pugh Class A or B). Administration of VIVJOA in patients with severe hepatic impairment (Child-Pugh Class C) has not been studied [see Clinical Pharmacology (12.3)].
9DESCRIPTION
VIVJOA (oteseconazole capsules) contains oteseconazole which is an oral azole antifungal agent.
The chemical name of oteseconazole is (R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(1H-tetrazol-1-yl)-1-(5-(4-(2,2,2-trifluoroethoxy)phenyl)pyridin-2-yl)propan-2-ol or 2-Pyridineethanol, α-(2,4-difluorophenyl)-β β-difluoro- α-(1H-tetrazol-1-ylmethyl)-5-(4-(2,2,2-trifluoroethoxy)phenyl)-,(αR)-. The empirical formula is C23H16F7N5O2. The molecular weight is 527.39 g/mol. The structural formula is
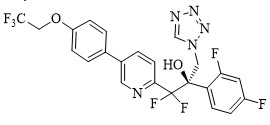
Oteseconazole is a white to off-white crystalline powder and is practically insoluble in water within a pH range of 1 to 9 but is soluble in a variety of organic solvents.
Each oteseconazole capsule, for oral use, contains 150 mg oteseconazole and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose, magnesium stearate, silicified microcrystalline cellulose, and sodium lauryl sulfate. Capsule shell and print constituents: FD&C Blue #1, FD&C Red #3, gelatin, Opacode SW-9008/SW-9009 and titanium dioxide. Contains no ingredient made from a gluten-containing grain (wheat, barley, or rye).
10CLINICAL PHARMACOLOGY
10.1Mechanism of Action
10.2Pharmacodynamics
Oteseconazole exposure-response relationships and the time course of pharmacodynamic response are unknown.
10.3Pharmacokinetics
The AUC of oteseconazole increased approximately dose proportionally while the Cmax increased less than dose proportionally over a dose range of 20 mg (0.13 times the lowest recommended dose) to 320 mg (0.53 times the highest recommended dose). The pharmacokinetic parameters of oteseconazole associated with the administration of the recommended dosing regimen of VIVJOA are presented in Table 1.
10.4Microbiology
11NONCLINICAL TOXICOLOGY
11.1Carcinogenesis, Mutagenesis, Impairment of Fertility
11.2Animal Toxicology and/or Pharmacology
In an oral carcinogenicity study, Sprague Dawley rats were administered doses of 0.5, 1.5, or 5 mg/kg/day oteseconazole once daily for up to 90 weeks. The high dose was initially reduced from 5 to 3 mg/kg/day in males due to excess mortality. Incidences of hemorrhage were increased in the adrenals, brain, coagulating gland, ears, epididymides, head, heart, lung, nose, pancreas, pharynx, prostate, seminal vesicles, spinal cord, testes, thymus, and bladder of male Crl:CD®(SD) rats (after 77 weeks of dosing at about 5 times the MRHD based on AUC comparisons). There were no increases in the incidence of hemorrhage in rats after 26 weeks at 5 mg/kg. The clinical relevance of these findings after very high doses (5 to 7 times the MRHD) for the lifetime of the rat remains unclear.
12CLINICAL STUDIES
13HOW SUPPLIED/STORAGE AND HANDLING
13.1How Supplied
VIVJOA (oteseconazole capsules) are supplied as lavender hard gelatin capsules. Printed black "OTE 150" on the capsule and contain 150 mg oteseconazole. They are available in an 18-count (NDC 74695-823-18) blister package within a child resistant wallet. There will be one blister pack per wallet and one wallet per outer carton.
The fluconazole/VIVJOA dosage regimen is in an 18-count (NDC 74695-945-18) blister package within a child resistant wallet. There is one blister pack of VIVJOA (oteseconazole capsules) per wallet and one wallet per outer carton. The outer carton and wallet contain the following: "fluconazole/VIVJOA dosage regimen" and "fluconazole is prescribed separately".
Fluconazole is not supplied in the carton.
13.2Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F to 86°F) [See USP Controlled Room Temperature]. Protect from light when removed from the outer carton.
14PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
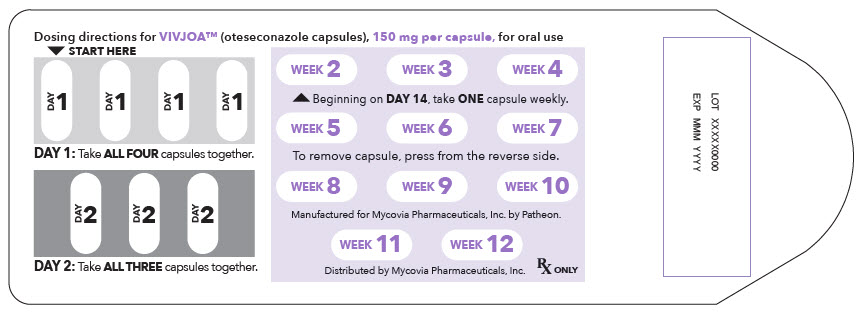
15PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack Label
Dosing directions for VIVJOA™ (oteseconazole capsules), 150 mg per capsule, for oral use
▼ START HERE
DAY
1
DAY
1
DAY
1
DAY
1
1
DAY
1
DAY
1
DAY
1
DAY 1: Take ALL FOUR capsules together.
DAY
2
DAY
2
DAY
2
2
DAY
2
DAY
2
DAY 2: Take ALL THREE capsules together.
WEEK
2
WEEK
3
WEEK
4
2
WEEK
3
WEEK
4
Beginning on DAY 14, take ONE capsule weekly.
WEEK
5
WEEK
6
WEEK
7
5
WEEK
6
WEEK
7
To remove capsule, press from the reverse side.
WEEK
8
WEEK
9
WEEK
10
8
WEEK
9
WEEK
10
Manufactured for Mycovia Pharmaceuticals, Inc. by Patheon.
WEEK
11
WEEK
12
11
WEEK
12
Distributed by Mycovia Pharmaceuticals, Inc.
Rx ONLY
LOT XXXXX0000
EXP MMM YYYY
EXP MMM YYYY
16PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack Container
NDC 74695-823-18
Rx only
Rx only
18 CAPSULES
Press and
hold button.
hold button.
vivjoa™
(oteseconazole capsules)
150 mg per capsule, for oral use
(oteseconazole capsules)
150 mg per capsule, for oral use
See package insert for full Prescribing Information.
STEP 1 Press and hold button.
STEP 2 Pull out medication card.
Pull out here.
▼
▼

17PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack Container Carton
NDC 74695-823-18
Rx only
Rx only
See package insert for full Prescribing Information.
vivjoa™
(oteseconazole capsules)
(oteseconazole capsules)
150 mg per capsule, for oral use
18 CAPSULES

Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?