Tabrecta
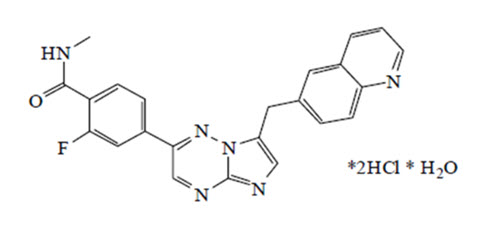
What is Tabrecta (Capmatinib)?
Approved To Treat
Related Clinical Trials
Summary: The hypothesize is that tepotinib is more effective than the investigator's choice of treatment in patients with MET-mutated NSCLC who have progressed after at least one first-line treatment. The main benefit concerns patient access to tepotinib. There is currently no access to a new-generation MET TKI in France for METex14 patients, due to lack of comparative data. There are no phase III RCTs und...
Summary: This phase II Lung-MAP treatment trial test the combination of targeted drugs (capmatinib, osimertinib, and/or ramucirumab) in treating patients with non-small cell lung cancer that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced) and that has EGFR and MET gene changes. Capmatinib and osimertinib are in a class of medications called...
Summary: DETERMINE is an open-label phase II/III trial. It will look at targeted treatments in rare cancers or common cancers with rare genetic change (mutation). Patients must have a cancer with an identified mutation. This could be found during routine testing or as part of another research programme. The DETERMINE trial will recruit adults, teenagers and children. If a drug is found to benefit a new pat...
Related Latest Advances
Brand Information
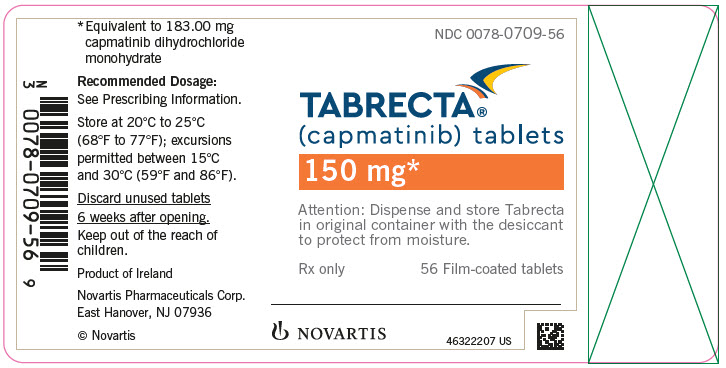
- 150 mg: pale orange brown, ovaloid, curved film-coated with beveled edges, unscored, debossed with ‘DU’ on one side and ‘NVR’ on the other side
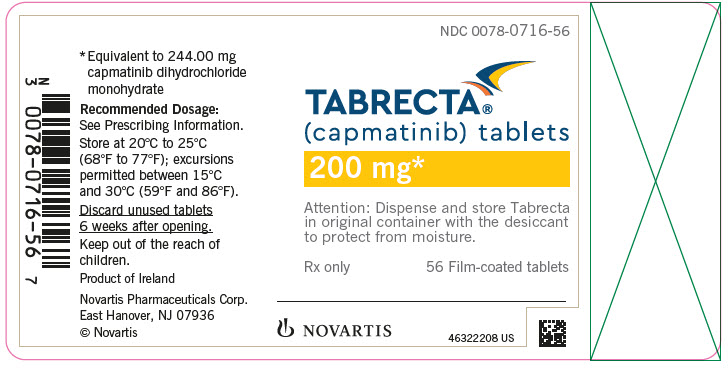
- 200 mg: yellow, ovaloid, curved film-coated with beveled edges, unscored, debossed with ‘LO’ on one side and ‘NVR’ on the other side
- ILD/Pneumonitis
- Hepatotoxicity
- Pancreatic Toxicity
- Hypersensitivity reactions