ELOQUENT-2 (NCT01239797)
The efficacy and safety of EMPLICITI in combination with lenalidomide and dexamethasone were evaluated in ELOQUENT-2, a randomized, open-label trial in patients with multiple myeloma who had received one to three prior therapies and had documented progression following their most recent therapy.
Eligible patients were randomized in a 1:1 ratio to receive either EMPLICITI in combination with lenalidomide and low-dose dexamethasone or lenalidomide and low-dose dexamethasone. Treatment was administered in 4-week cycles until disease progression or unacceptable toxicity. EMPLICITI 10 mg/kg was administered intravenously each week for the first 2 cycles and every 2 weeks thereafter. Prior to EMPLICITI infusion, dexamethasone was administered as a divided dose: an oral dose of 28 mg and an intravenous dose of 8 mg. In the control group and on weeks without EMPLICITI, dexamethasone 40 mg was administered as a single oral dose weekly. Lenalidomide 25 mg was taken orally once daily for the first 3 weeks of each cycle. Assessment of tumor response was conducted every 4 weeks.
A total of 646 patients were randomized to receive treatment: 321 to EMPLICITI in combination with lenalidomide and low-dose dexamethasone and 325 to lenalidomide and low-dose dexamethasone.
Demographics and baseline disease characteristics were balanced between treatment arms. The median age was 66 years (range, 37-91); 57% of patients were 65 years or older; 60% of patients were male; whites comprised 84% of the study population, Asians 10%, and blacks 4%. The ECOG performance status was 0 in 47%, 1 in 44%, and 2 in 9% of patients, and ISS Stage was I in 43%, II in 32%, and III in 21% of patients. The cytogenetic categories of del 17p and t(4;14) were present in 32% and 9% of patients, respectively. The median number of prior therapies was 2. Thirty-five percent (35%) of patients were refractory (progression during or within 60 days of last therapy) and 65% were relapsed (progression after 60 days of last therapy). Prior therapies included stem cell transplant (55%), bortezomib (70%), melphalan (65%), thalidomide (48%), and lenalidomide (6%).
The efficacy of EMPLICITI was evaluated by progression-free survival (PFS) as assessed by hazard ratio, and overall response rate (ORR) as determined by a blinded Independent Review Committee using the European Group for Blood and Marrow Transplantation (EBMT) response criteria. Efficacy results are shown in Table 12 and Figure 1. The median number of treatment cycles was 19 for the EMPLICITI group and 14 for the comparator arm with a minimum follow-up of two years.
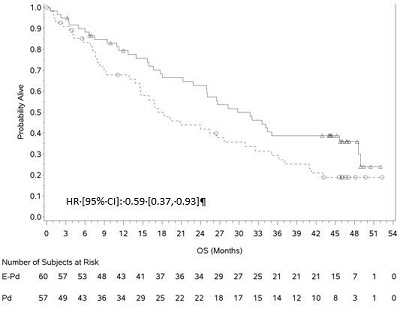
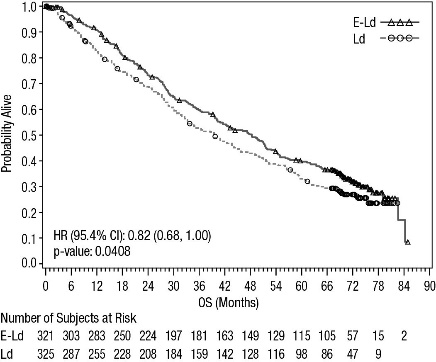
A pre-planned final overall survival (OS) analysis was performed after at least 427 deaths occurred. The minimum follow-up was 70.6 months. The OS results at final analysis reached statistical significance. A significantly longer OS was observed in patients in the E-Ld arm compared to patients in Ld arm, representing an 18% reduction in the risk of death. Efficacy results are presented in Table 12 and Figure 2.
Figure 1: ELOQUENT-2 Progression-Free Survival
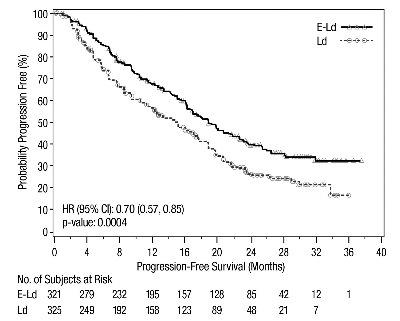
The 1- and 2-year rates of PFS for EMPLICITI in combination with lenalidomide and dexamethasone treatment were 68% and 41%, respectively, compared with 57% and 27%, respectively, for lenalidomide and dexamethasone treatment.
Figure 2: ELOQUENT-2 Overall Survival
ELOQUENT-3 (NCT02654132)
The efficacy and safety of EMPLICITI in combination with pomalidomide and dexamethasone were evaluated in ELOQUENT-3, a randomized, open-label trial in patients with relapsed or refractory multiple myeloma.
Eligible patients were randomized in a 1:1 ratio to receive either EMPLICITI in combination with pomalidomide and low-dose dexamethasone or pomalidomide and low-dose dexamethasone. Treatment was administered in 4-week cycles until disease progression or unacceptable toxicity. EMPLICITI 10 mg/kg was administered intravenously each week for the first 2 cycles and 20 mg/kg every 4 weeks thereafter.
Prior to EMPLICITI infusion, dexamethasone was administered. Dexamethasone was administered on day 1, 8, 15 and 22 of each cycle. On weeks with EMPLICITI infusion, dexamethasone was administered as a divided dose: subjects 75 years or younger, an oral dose of 28 mg and an intravenous dose of 8 mg, and in subjects older than 75 years an oral dose of 8 mg and an intravenous dose of 8 mg. On weeks without an EMPLICITI infusion and in the control group, dexamethasone was administered in subjects 75 years or younger as an oral dose of 40 mg and in subjects older than 75 years as an oral dose of 20 mg dexamethasone was administered orally. Assessment of tumor response was conducted every 4 weeks.
A total of 117 patients were randomized to receive treatment: 60 to EMPLICITI in combination with pomalidomide and low-dose dexamethasone and 57 to pomalidomide and low-dose dexamethasone.
Demographics and baseline disease characteristics were balanced between treatment arms. The median age was 67 years (range, 36-81); 62% of patients were 65 years or older; 57% of patients were male; whites comprised 77% of the study population, Asians 21%, and blacks 1%. The ECOG performance status was 0 in 44%, 1 in 46%, and 2 in 10% of patients, and ISS Stage was I in 50%, II in 38%, and III in 12% of patients. The chromosomal lab abnormalities as determined by FISH of del 17p and t(4;14) were present in 5% and 11% of patients, respectively. The median number of prior therapies was 3. Eighty-seven percent (87%) of patients were refractory to lenalidomide, 80% refractory to a proteasome inhibitor, 70% were refractory to both lenalidomide and a proteasome inhibitor. Prior therapies included stem cell transplant (55%), bortezomib (100%), lenalidomide (99%), cyclophosphamide (66%), melphalan (63%), carfilzomib (21%), and daratumumab (3%).
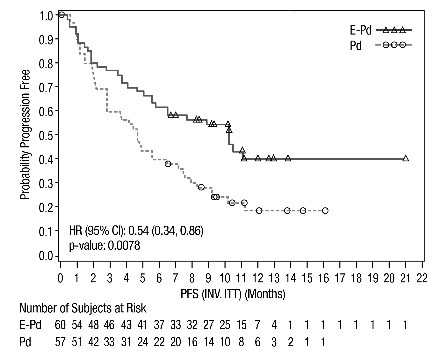
The efficacy of EMPLICITI was evaluated by progression-free survival (PFS) and overall response rate (ORR) as determined by the investigator. Efficacy results are shown in Table 13 and Figure 3. The median number of treatment cycles was 9 for the EMPLICITI group and 5 for the comparator arm with a minimum follow-up of 9.1 months.
A pre-planned final OS analysis was performed after at least 78 deaths occurred. The minimum follow-up was 45.0 months. A longer OS was observed in patients in the E-Pd arm compared to patients in the Pd arm. Efficacy results are presented in Table 13 and Figure 4.
Figure 3: ELOQUENT-3 Progression-Free Survival
Figure 4: ELOQUENT-3 Overall Survival