Tazverik
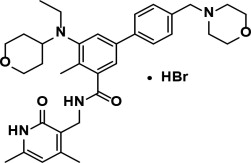
What is Tazverik (Tazemetostat)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This phase I trial tests the safety, side effects, and best dose of tazemetostat in combination with topotecan and pembrolizumab in treating patients with small cell lung cancer that has come back after a period of improvement (recurrent). Tazemetostat may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Chemotherapy drugs, such as topotecan, work in different...
Summary: The participants of this study would have relapsed/refractory follicular lymphoma. Follicular lymphoma is a type of blood cancer. It is referred to as 'relapsed' when the disease has come back after a period of improvement after that follows a treatment regimen and 'refractory' when treatment no longer works. Stage 1 of this trial will study the safety and the level that adverse effects of each of...
Summary: The purpose of this clinical trial is to learn if the study drug Tazemetostat combined with Zanubrutinib and anti-CD20 monoclonal antibody is safe and effective in treating patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma.
Related Latest Advances
Brand Information
- Secondary Malignancies
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma.


