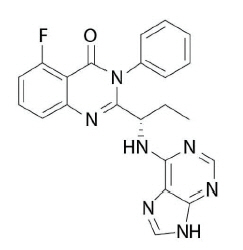
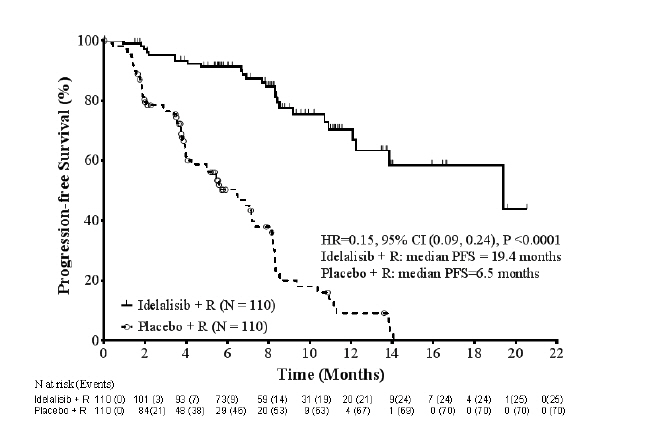
Zydelig
What is Zydelig (Idelalisib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to investigate the efficacy and safety of BGB-16673 compared with investigator's choice (idelalisib plus rituximab \[for CLL only\] or bendamustine plus rituximab or venetoclax plus rituximab retreatment) in participants with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) previously exposed to both BTK inhibitors (BTKi) and BCL2 inhibitors (BCL2...
Summary: This is a phase 3, open-label, randomized, multi-center study assessing the efficacy and safety of DZD8586 versus investigator's choice in participants with chronic lymphocytic leukemia/small lymphocytic lymphoma who have progressed following prior therapy. Primary objective of this study is to assess the efficacy using progression free survival assessed by independent review committee as primary ...
Summary: The purpose of the study is to investigate the effect and side effects of personalized cancer treatment in patients with metastatic colorectal cancer (bowel cancer). All patients included must have metastatic bowel cancer and receive or have received at least two lines of standard chemotherapy. The cancer must not be available for surgery with curative intent.
Related Latest Advances
Brand Information
- 100 mg: orange, oval-shaped, film-coated tablet debossed with "GSI" on one side and "100" on the other side.
- 150 mg: pink, oval-shaped, film-coated tablet debossed with "GSI" on one side and "150" on the other side.
- Hepatotoxicity
- Severe Diarrhea or Colitis
- Pneumonitis
- Infections
- Intestinal Perforation
- Severe Cutaneous Reactions
- Hypersensitivity Reactions
- Neutropenia