Brand Name
Cerezyme
Generic Name
Imiglucerase
View Brand Information FDA approval date: May 23, 1994
Classification: Hydrolytic Lysosomal Glucocerebroside-specific Enzyme
Form: Injection
What is Cerezyme (Imiglucerase)?
Cerezyme is indicated for treatment of adults and pediatric patients 2 years of age and older with Type 1 Gaucher disease that results in one or more of the following conditions: anemia thrombocytopenia bone disease hepatomegaly or splenomegaly Cerezyme is a hydrolytic lysosomal glucocerebrosidase-specific enzyme indicated for treatment of adults and pediatric patients 2 years of age and older with Type 1 Gaucher disease that results in one or more of the following conditions: anemia, thrombocytopenia, bone disease, hepatomegaly or splenomegaly.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Gaucher Disease Registry Protocol
Summary: The ICGG Gaucher Registry is an ongoing, international multi-center, strictly observational program that tracks the routine clinical outcomes for patients with Gaucher disease, irrespective of treatment status. No experimental intervention is involved; patients in the Registry undergo clinical assessments and receive care as determined by the patient's treating physician. The objectives of the Reg...
Related Latest Advances
Brand Information
Cerezyme (IMIGLUCERASE)
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Initiate CEREZYME in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue CEREZYME and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur
1INDICATIONS AND USAGE
CEREZYME is indicated for the treatment of non-central nervous system (CNS) manifestations of Type 1 or Type 3 Gaucher disease in adults and pediatric patients.
2DOSAGE FORMS AND STRENGTHS
For injection: 400 units of imiglucerase as a white to off-white lyophilized powder in a single-dose vial for reconstitution.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions Including Anaphylaxis
- Infusion-Associated Reactions
4.1Clinical Trials and Postmarketing Experience
The following adverse reactions associated with the use of imiglucerase were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
See Table 2 for adverse reactions occurring in adults and pediatric patients treated with CEREZYME in clinical trials and the postmarketing setting.
4.2Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of CEREZYME or of other imiglucerase products.
Approximately 15% of patients treated and tested to date have developed IgG antibody to CEREZYME during the first year of therapy. Patients who developed IgG antibody did so largely within 6 months of treatment and rarely developed antibodies to CEREZYME after 12 months of therapy. Approximately 46% of patients with detectable IgG antibodies experienced symptoms of hypersensitivity. Patients with antibody to CEREZYME have higher risk of hypersensitivity reaction
5DESCRIPTION
Imiglucerase is a hydrolytic lysosomal glucocerebrosidase-specific enzyme. It is an analogue of the human enzyme b-glucocerebrosidase (b-D-glucosyl-N-acylsphingosine glucohydrolase, E.C. 3.2.1.45), produced by recombinant DNA technology using mammalian cell culture (Chinese hamster ovary). Purified imiglucerase is a monomeric glycoprotein of 497 amino acids, containing 4 N-linked glycosylation sites (Mr=60,430). Imiglucerase differs from placental glucocerebrosidase by one amino acid at position 495, where histidine is substituted for arginine. The oligosaccharide chains at the glycosylation sites have been modified to terminate in mannose sugars. The modified carbohydrate structures on imiglucerase are somewhat different from those on placental glucocerebrosidase.
CEREZYME (imiglucerase) for injection is intended for intravenous use. It is supplied as a sterile, nonpyrogenic, white to off-white lyophilized powder for reconstitution with Sterile Water for Injection, USP. Each single-dose vial contains 424 units imiglucerase, mannitol (340 mg), polysorbate 80, NF (1.06 mg), and sodium citrates: disodium hydrogen citrate (36 mg) and trisodium citrate (104 mg).
An enzyme unit (U) is defined as the amount of enzyme that catalyzes the hydrolysis of 1 micromole of the synthetic substrate para-nitrophenyl-b-D-glucopyranoside (pNP-Glc) per minute at 37°C. Reconstituted solutions have a pH of approximately 6.1.
6CLINICAL STUDIES
Study 1 was a randomized, double-blind, active-controlled clinical trial that enrolled 30 patients (17 male and 13 female) with Type 1 Gaucher disease. Patient ages ranged from 12 to 69 years, with a mean age of 38 years in the CEREZYME treatment group and a mean age of 28 years in the alglucerase treatment group at baseline. The inclusion criteria required patients to have a hemoglobin level of at least 1 g/dL below the lower limit of the normal range for their respective age and sex. Patients were randomized 1:1 to receive either CEREZYME 60 units/kg administered intravenously every other week or alglucerase for 6 months.
In Study 1, the primary efficacy parameters were an increase in hemoglobin concentration (at least 1 g/dL) and platelet count and a decrease in spleen and liver volume at 6 months. Efficacy results are shown in Table 3.
In Study 1, bone x-rays showed improvements in cortical thickness and lucencies in 7 of 11 CEREZYME-treated patients.
In Study 2, an open-label extension study of Study 1, 29 patients with Type 1 Gaucher disease continued their assigned treatment for an additional 18 months. Patients were unblinded 3 months into Study 2 and those on alglucerase were allowed to cross-over to CEREZYME treatment. After total treatment duration of CEREZYME for 18–24 months, mean increase from baseline of Study 1 in hemoglobin was 2.4 g/dL, mean increase in platelet count was 40 ×10
The efficacy of CEREZYME for the treatment of non-CNS manifestations of Type 1 and Type 3 Gaucher disease was assessed in Study 3, an observational study, using data from the International Collaborative Gaucher Group (ICGG) Gaucher Disease Registry (NCT00358943). Study 3 included patients with Type 1 or Type 3 Gaucher disease who were treated with CEREZYME as initial therapy with an index clinical assessment and one or more follow-up clinical assessments. Study 3 was a baseline-controlled analysis in patients with Type 1 Gaucher disease (19 weeks to 87 years of age) and patients with Type 3 Gaucher disease (7 weeks to 54 years of age) who received CEREZYME intravenously as prescribed by their physicians (initiated treatment between 1992–2021). After approximately two years (1 to 3 years) of CEREZYME treatment in patients with Type 1 and 3 Gaucher disease, mean changes from baseline in the following measures showed improvement: hemoglobin, platelet count, liver volume, spleen volume, and height Z-score.
- Among 1,052 Type 1 Gaucher disease patients, mean baseline hemoglobin was 11.8 g/dL and mean increase from baseline was 1.5 g/dL (95% CI: 1.4, 1.5).
- Among 1,053 Type 1 Gaucher disease patients, mean baseline platelet count was 128×10
- Among 118 Type 3 Gaucher disease patients, mean baseline hemoglobin levels were 10 g/dL and mean increase from baseline was 1.8 g/dL (95% CI: 1.5, 2.1).
- Among 116 Type 3 Gaucher disease patients, mean baseline platelet count was 149×10
The 2-year summaries include measurements within 1 to 3 years after treatment initiation due to the lack of predefined data collection timepoints in the registry.
7HOW SUPPLIED/STORAGE AND HANDLING
CEREZYME (imiglucerase) for injection is supplied as a white to off-white lyophilized powder in a carton containing one single-dose vial: NDC 58468-4663-1. Each vial contains 400 units of imiglucerase. CEREZYME does not contain any preservatives.
8PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions Including Anaphylaxis and Infusion-Associated Reactions
Advise patients and caregivers that life-threatening hypersensitivity reactions, including anaphylaxis, and IARs may occur with CEREZYME treatment.
Advise patients and caregivers that anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Inform patients and caregivers of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis, and IARs and to seek immediate medical care should symptoms occur
Patient Registry
Inform patients and caregivers that the Gaucher patient registry has been established in order to better understand the variability and progression of Gaucher disease and to continue to monitor and evaluate long-term treatment effects of CEREZYME. A pregnancy sub-registry will also monitor the effects of CEREZYME on pregnant women and their offspring
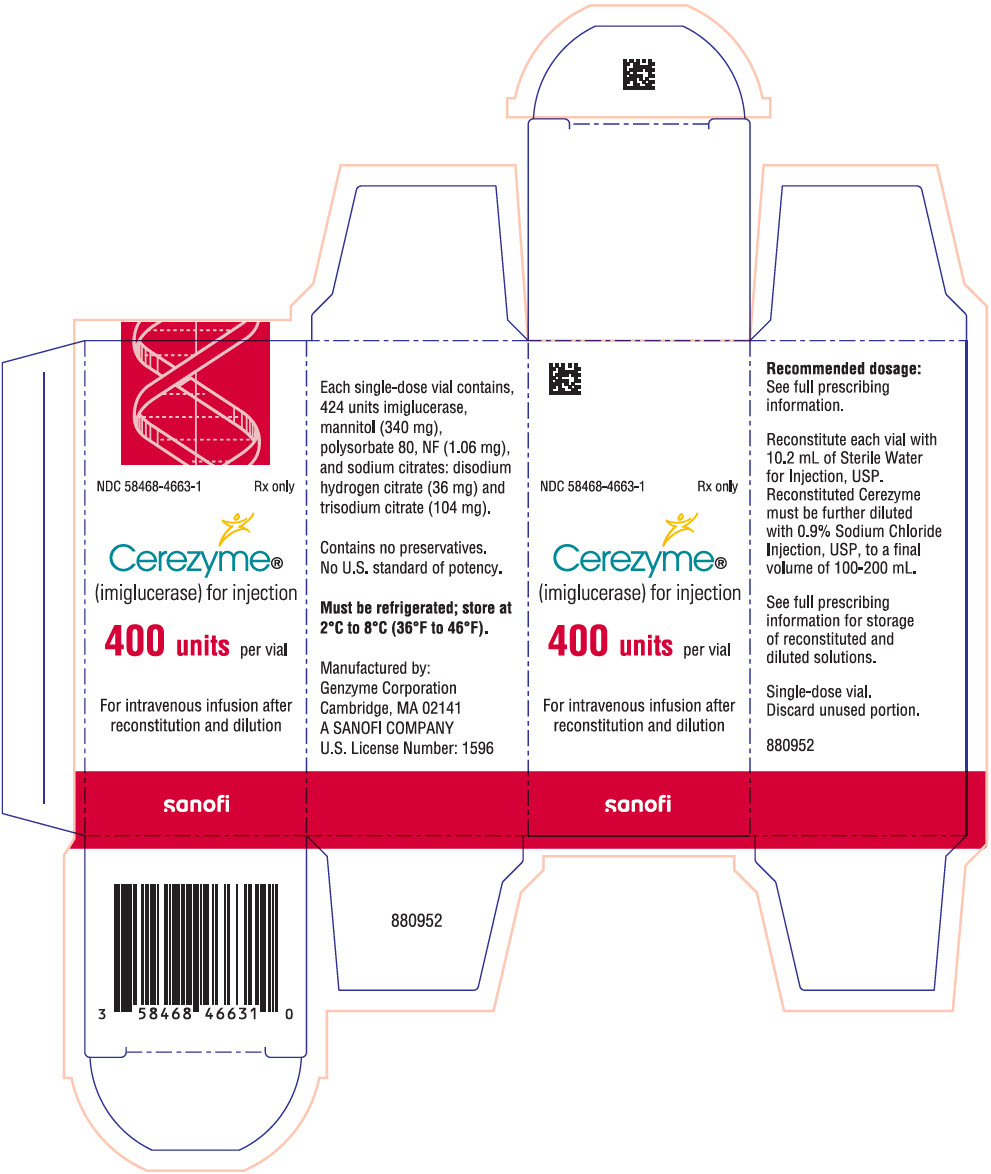
9PRINCIPAL DISPLAY PANEL - 400 Unit Vial Carton
NDC 58468-4663-1
Cerezyme
400 units per vial
For intravenous infusion after
sanofi