Nexletol
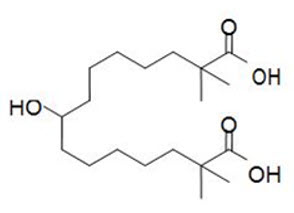
What is Nexletol (Bempedoic)?
Approved To Treat
Related Clinical Trials
Summary: This is a randomized placebo-controlled study in treated and suppressed HIV-infected individuals aged ≥40 years with either known CVD or 1 CVD risk factor to study the effect of Bempedoic acid (BA) on safety, arterial inflammation as assessed by FDG-PET/CT, lipids, inflammation, immune activation, cardiometabolic indices, and non-calcified plaque (NCP) in the coronary arteries (assessed by coronar...
Summary: Data on the real-world use and effectiveness and safety of bempedoic acid combined with both a statin and ezetimibe in clinical practice is limited. There is an increased focus on using combination therapy to lower LDL-C.
Summary: Monotherapies for lowering LDL-C often do not achieve target lipid levels because they act on a single pathway, which may be insufficient in patients with high cardiovascular risk or complex lipid profiles. Triple combination therapies, targeting multiple mechanisms of cholesterol metabolism simultaneously, have demonstrated superior LDL-C reduction and better achievement of guideline recommended ...
Related Latest Advances
Brand Information
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, myocardial infarction, stroke, or coronary revascularization) in adults at increased risk for these events who are unable to take recommended statin therapy (including those not taking a statin).
- as an adjunct to diet and exercise, in combination with other low-density lipoprotein cholesterol (LDL-C) lowering therapies or alone when concomitant LDL-C lowering therapy is not possible, to reduce LDL-C in adults with hypercholesterolemia, including heterozygous familial hypercholesterolemia (HeFH).
- Tablets: 180 mg, white to off-white, oval shaped, debossed with "180" on one side and "ESP" on the other side.
- Hyperuricemia
- Tendon Rupture