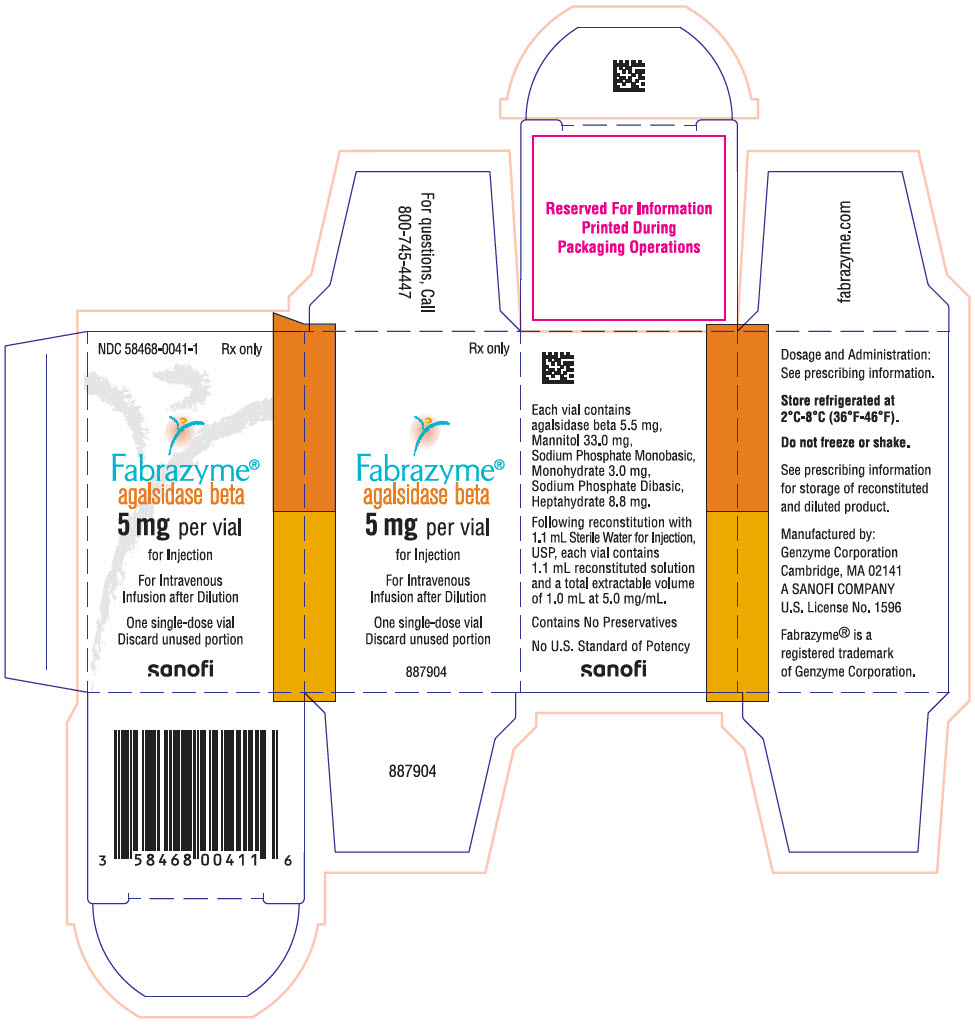
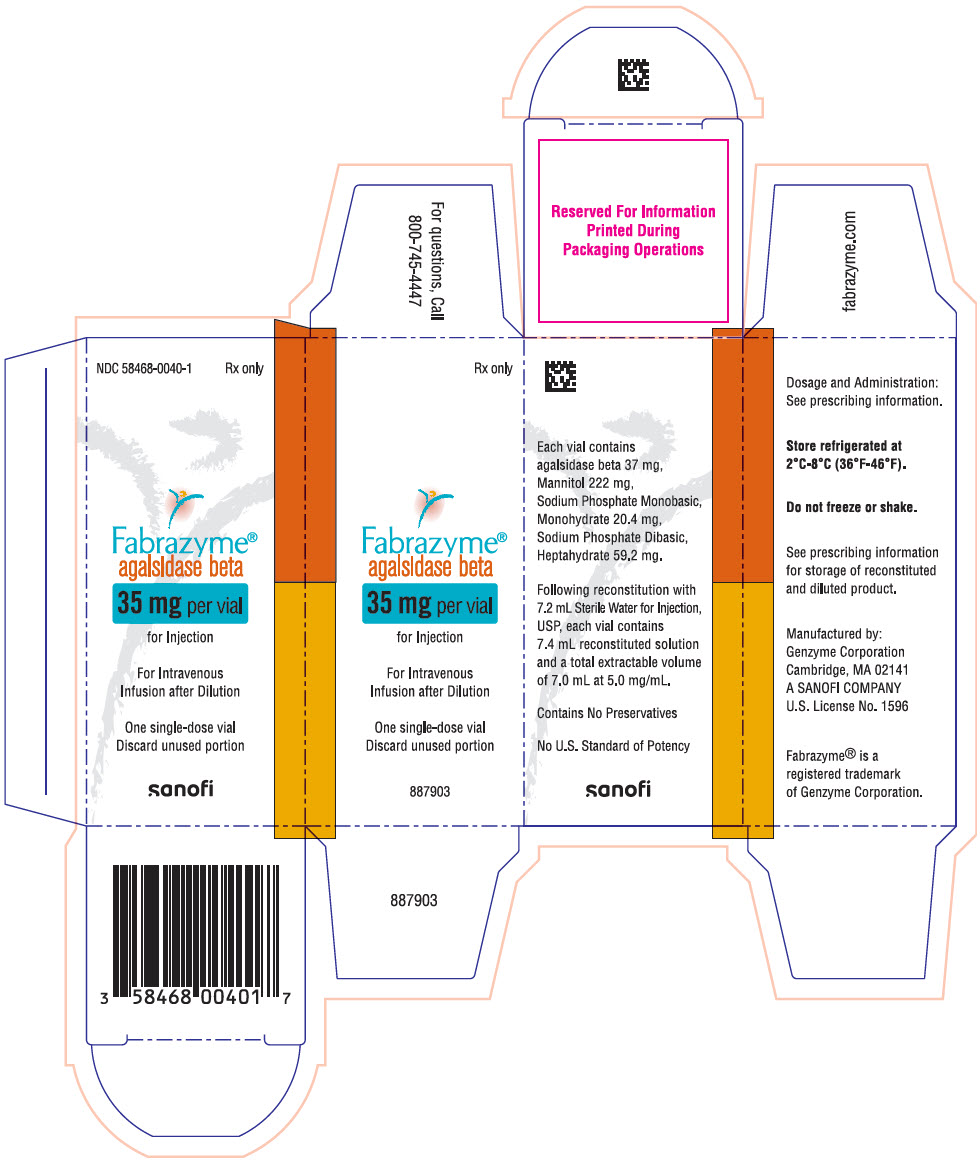
Fabrazyme
What is Fabrazyme (Agalsidase)?
Approved To Treat
Related Clinical Trials
Summary: This is a prospective observational study. All patients will initiate and maintain treatment with agalsidase alfa during the study period. All patients will receive a full standard of care concomitant medication for the treatment of their cardiac condition. Twenty-five patients with genetically confirmed Anderson-Fabry disease will undergo PET-CMR at baseline and after 12 months of treatment with ...
Summary: The Fabry Registry is an ongoing, international multi-center, strictly observational program that tracks the routine clinical outcomes for patients with Fabry disease, irrespective of treatment status. No experimental intervention is involved; patients in the Registry undergo clinical assessments and receive care as determined by the patient's treating physician. The primary objectives of the Regi...
Summary: The main aim of this study is to learn more about the safety profile of Replagal. Participants will receive Replagal every 2 weeks at the clinic for about 1 year.
Related Latest Advances
Brand Information
- Hypersensitivity Reactions Including Anaphylaxis
- Infusion-Associated Reactions
- Cardiovascular: cardiorespiratory arrest, cardiac failure, myocardial infarction, palpitations
- Hypersensitivity reactions: anaphylaxis [see , localized angioedema (including auricular swelling, eye swelling, dysphagia, lip swelling, edema, pharyngeal edema, face swelling, and swollen tongue), and bronchospasm
- General: hyperhidrosis, asthenia, infusion site reaction
- Lymphatic: lymphadenopathy
- Musculoskeletal: arthralgia
- Neurologic: cerebrovascular accident, hypoesthesia, oral hypoesthesia
- Pulmonary: respiratory failure, hypoxia
- Renal: renal failure
- Vascular: leukocytoclastic vasculitis