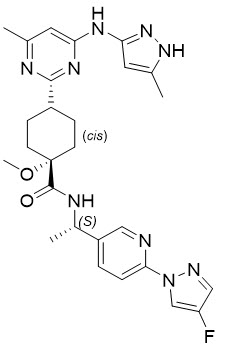
Gavreto
What is Gavreto (Pralsetinib)?
Approved To Treat
Related Clinical Trials
Summary: TAPISTRY is a Phase II, global, multicenter, open-label, multi-cohort study designed to evaluate the safety and efficacy of targeted therapies or immunotherapy as single agents or in rational, specified combinations in participants with unresectable, locally advanced or metastatic solid tumors determined to harbor specific oncogenic genomic alterations or who are tumor mutational burden (TMB)-high...
Summary: This trial will evaluate the efficacy and safety of various therapies in participants with Stage IB, IIA, IIB, IIIA, or selected IIIB resectable and untreated NSCLC tumors that meet protocol-specified biomarker criteria.
Summary: This study will evaluate the efficacy and safety of multiple therapies in participants with locally advanced, unresectable, Stage III NSCLC with eligible biomarker status as determined by Version 8 of the American Joint Committee on Cancer/Union for International Cancer Control NSCLC staging system.
Related Latest Advances
Brand Information
- Interstitial Lung Disease/Pneumonitis
- Hypertension
- Hepatotoxicity
- Hemorrhagic Events
- Tumor Lysis Syndrome
- Risk of Impaired Wound Healing
- Embryo-Fetal Toxicity
- Bottles of 60 capsules (NDC 71332-006-60).
- Bottles of 90 capsules (NDC 71332-006-90).
- Bottles of 120 capsules (NDC 71332-006-12).
(pralsetinib)
capsules

(pralsetinib)
capsules



